
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.
#Carbon disulfide formula how to
This site explains how to find molar mass. The reason is that the molar mass of the substance affects the conversion. To complete this calculation, you have to know what substance you are trying to convert. These relative weights computed from the chemical equation are sometimes called equation weights.Ī common request on this site is to convert grams to moles. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. Physik., 1939, 113, 710-720.In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Über die spezifische Wärme des Äthyläthers, des Nitrobenzols und des Schwefelkohlenstoffs, On the thermal capacity of some pure liquids and azeotropic mixtures, Some thermodynamice properties of the systems benzene + ethylene dichloride, benzene + carbon tetrachloride, acetone + chloroform, and acetone + carbon disulphide, The entropy and heat of fusion of carbon disulfide, The heat capacity of carbon disulfide from 15 to 300°K. Thermochemistry of Organic and Organometallic Compounds, Academic Press, New York, 1970, 1-636. Sur la chaleur de combustion du sulfure de carbon,Ĭompt. Methanethiol and carbon disulfide: Heats of combustion and formation by rotating-bomb calorimetry, NIST-JANAF Themochemical Tables, Fourth Edition, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Notes SRD 156 – Clathrate Hydrate Physical Property Database
#Carbon disulfide formula professional
SRSD 3 – Web Thermo Tables (WTT), professional edition SRSD 2 – Web Thermo Tables (WTT), "lite" edition SRD 103b – Thermo Data Engine (TDE) for pure compounds, SRD 103a – Thermo Data Engine (TDE) for pure compounds. (TRC) data available from this site, much more physicalĪnd chemical property data is available from the In addition to the Thermodynamics Research Center ACĮnthalpy of vaporization (at saturation pressure)Ĭoefficents calculated by NIST from author's data. See also Stephenson and Malanowski, 1987. Uncertainty assigned by TRC = 0.02 K TRCĪverage of 6 values Individual data pointsīased on data from 255. ChickosĪverage of 13 out of 14 values Individual data pointsĪverage of 8 values Individual data points
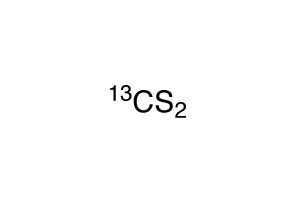
TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny directorĪC - William E. Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, References, Notes Reanalyzed by Cox and Pilcher, 1970, Original value = -1682.3 ± 0.50 kJ/mol ALSĬonstant pressure heat capacity of liquid C p,liquid (J/mol*K) Go To: Top, Gas phase thermochemistry data, Phase change data, References, NotesĭH - Eugene S. Requires a JavaScript / HTML 5 canvas capable browser. Gas Phase Heat Capacity (Shomate Equation) Secretary of Commerce on behalf of the U.S.A.ĪLS - Hussein Y. Go To: Top, Condensed phase thermochemistry data, Phase change data, References, Notesīy the U.S.

Your institution may already be a subscriber.įollow the links above to find out more about the data With the development of data collections included in The purpose of the fee is to recover costs associated NIST subscription sites provide data under theĭata Program, but require an annual fee to access.

Computational Chemistry Comparison and Benchmark Database.Electron-Impact Ionization Cross Sections (on physics web site).Use this link for bookmarking this species This structure is also available as a 2d Mol file IUPAC Standard InChIKey: QGJOPFRUJISHPQ-UHFFFAOYSA-N Copy.


 0 kommentar(er)
0 kommentar(er)
